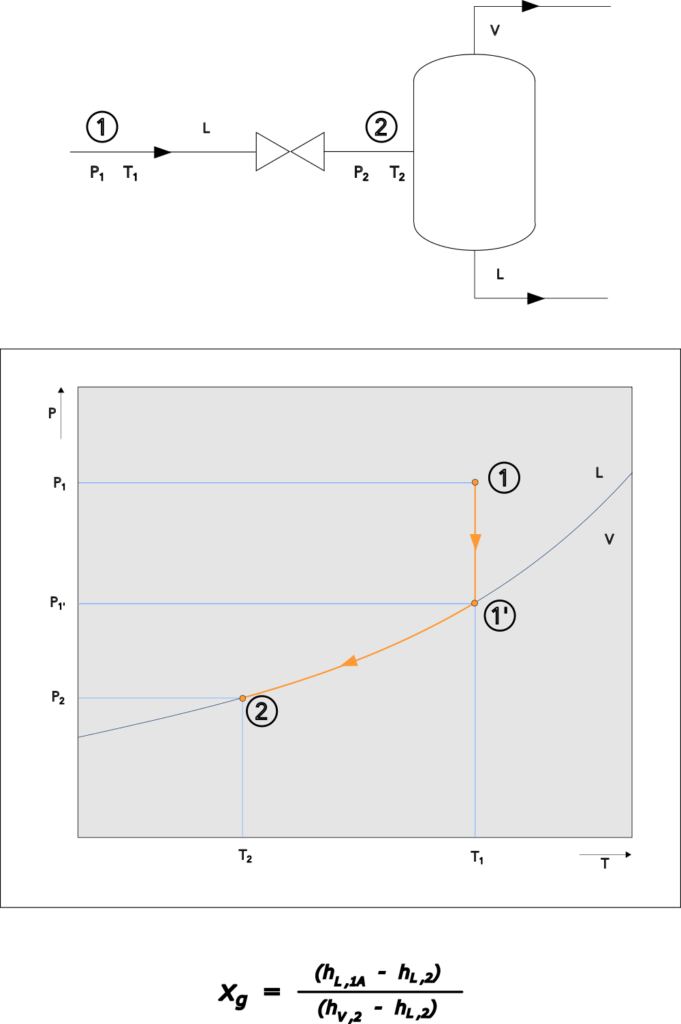
Adiabatic Flashing Liquid
A liquid at a high pressure, let down to a lower pressure will flash when the boiling temperature at the low pressure is lower than the temperature of the liquid. This can occur in valves isolating a higher pressure section from a lower pressure section but also in safety relief valves safeguarding high pressure liquid filled systems with a relief to a low pressure system or to atmosphere.
For single component systems an estimation for the flashing vapor can be done based on the energy balance. So as an example assume a liquid with T1 and P1 flashing adiabatic over a valve to a lower pressure P2 as shown in below illustration. With decreasing pressure the temperature of the liquid will stay unchanged until the boiling point is reached (1′), at this point, when reducing the pressure further, vapor will form and the heat of evaporation will reduce the temperature of the system. Reducing the pressure further will increase the amount of vapor formed and reduce the temperature according the VLE line in the P-T diagram for hat component until the pressure P2 is reached.
In case the difference between P1 and P2 in combination with the temperature T1 is such that allong the depressurization all liquid is evaporated the flash is 100%.