Sustainable Fertilizer Production
Through the well known Haber-Bosch route
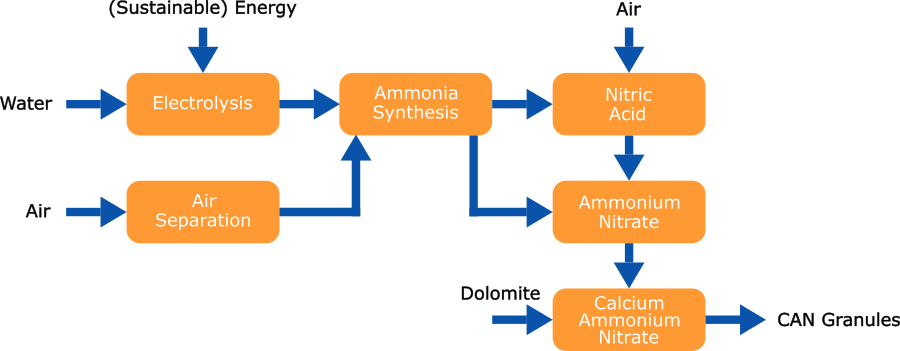
The initial option that comes to mind when considering sustainable fertilizer production is the production of hydrogen through electrolysis rather than, for instance, steam reforming of natural gas. The green hydrogen generated can subsequently be processed into fertilizers using well-established technologies that can be specifically optimized for this purpose. For instance, this approach can be utilized to produce green calcium ammonium nitrate.
Although based on existing technology this route is not without challenges, both technical and economically like:
- the produced ammonia OPEX is high, due to the high energy demand of electrolysis, resulting in a high OPEX for the produced fertilizer
- flexibilities (operating window) of the process sections are not equal, to accommodate fluctuating power availability intermediate storage needs to be well designed, including potentially power back up or power storage.
It also still has opportunities to optimize, thinking about the new developments in SOEC electrolysis, the steam produced in the ammonia loop and in the nitric acid plant can be very efficiently utilized to lower the energy consumption of the total plant. Utilizing the produced high pressure steam in SOEC is more efficient than using it, as traditionally is done, to drive compressors over a steam turbine.
Syngas
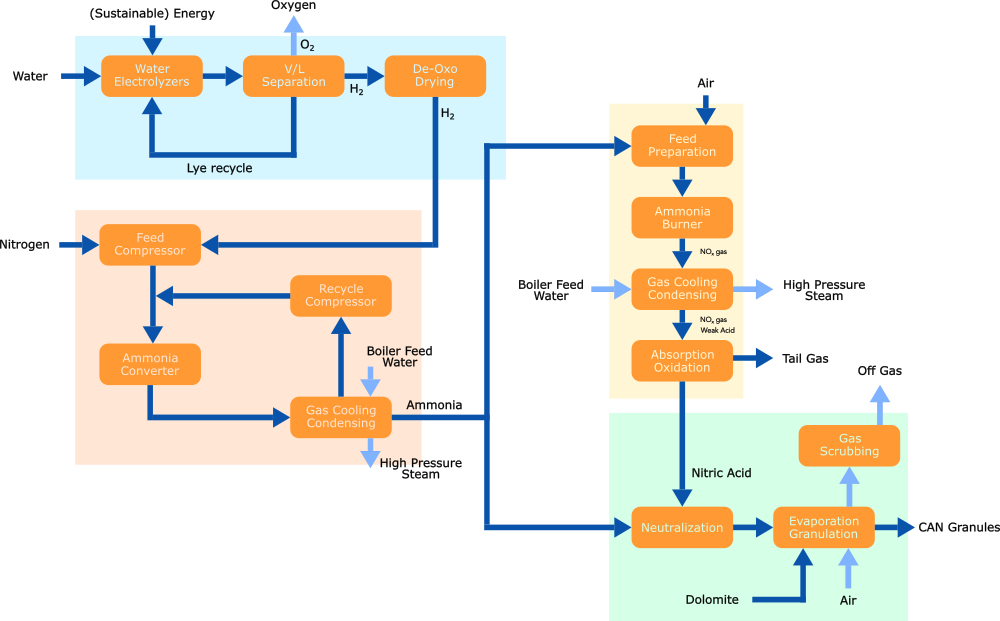
Synthesis Gas, commonly known as Syngas, serves as the feedstock for ammonia production in the fertilizer industry. It is a gaseous mixture composed of nitrogen (N2) and hydrogen (H2) in a molar ratio of H2:N2 of 3:1.
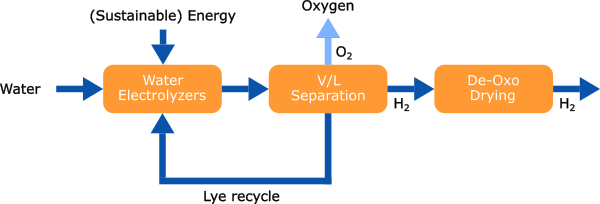
Hydrogen is generated through alkaline water electrolysis (AWE), followed by purification processes involving de-oxidation and drying. Nitrogen is extracted from the atmosphere via an air separation unit (ASU) and may undergo further purification. Subsequently, nitrogen and hydrogen are combined in the desired molar ratio and compressed to the pressure required for ammonia synthesis.
Synthesis Gas, commonly known as Syngas, serves as the feedstock for ammonia production in the fertilizer industry. It is a gaseous mixture composed of nitrogen (N2) and hydrogen (H2) in a molar ratio of H2:N2 of 3:1.
Hydrogen is generated through alkaline water electrolysis (AWE), followed by purification processes involving de-oxidation and drying. Nitrogen is extracted from the atmosphere via an air separation unit (ASU) and may undergo further purification. Subsequently, nitrogen and hydrogen are combined in the desired molar ratio and compressed to the pressure required for ammonia synthesis.
Ammonia
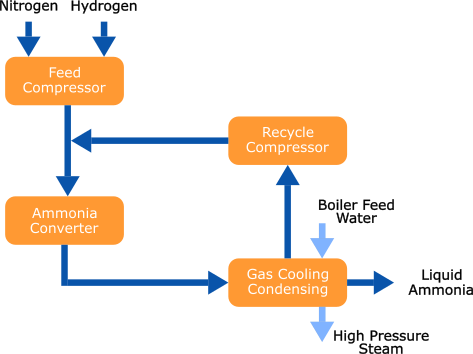
The syngas is compressed to the necessary (Haber-Bosch) ammonia synthesis loop pressure, where ammonia (NH3) is produced over a catalyst in the ammonia converter. The resulting gas mixture is subsequently cooled to condense ammonia and separate it from the hydrogen and nitrogen gas streams. The liquid ammonia is transferred to an intermediate storage, and the unconverted nitrogen and hydrogen are recycled back to the Ammonia converter, mixed with the fresh feed.
Nitric Acid
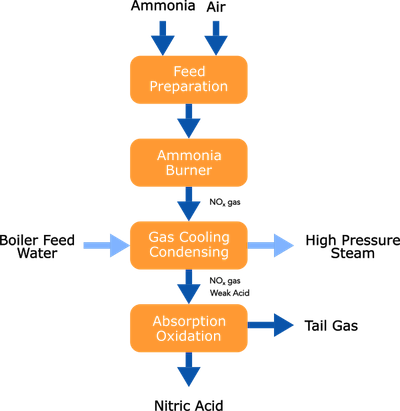
Nitric acid is produced from ammonia utilizing the Ostwald process in either dual or mono-pressure form depending on required plant size.
Ammonia is evaporated and filtered, then mixed with air and fed to an ammonia burner. Over Platinum catalysts, ammonia is oxidized to predominantly Nitric oxide (NO). The NO-rich gas is cooled first by generating high-pressure steam and process-process heat exchange, and later by utilizing cooling water. While cooling the nitric oxide, it will convert into nitric dioxide, which, once the dew point is reached, will absorb in water and form nitric acid (HNO3). After a final absorption/oxidation step, the remaining NOx and N2O are removed from the tail gas in an abatement reactor before being released to the atmosphere. The nitric acid solution produced is typically in the 58-65% wt. range.
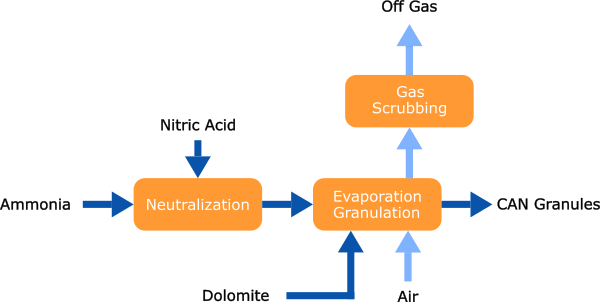
Calcium Ammonium Nitrate
The nitric acid solution is now neutralized using ammonia, resulting in a solution of ammonium nitrate. This solution is concentrated in an evaporation section and subsequently mixed with dolomite, thereby enhancing the stability and safety of the product. The resulting mixture is then processed into final fertilizer grade granules.